Historically, medical trials not solely place a big burden on sufferers and individuals because of the prices related to transportation, lodging, meals, and dependent care, but in addition have an environmental influence. With the development of obtainable applied sciences, decentralized medical trials have grow to be a extensively widespread subject of dialogue and supply a extra sustainable method. Decentralized medical trials cut back the necessity to journey to review websites by reducing the monetary burden on all events concerned, thereby accelerating affected person recruitment and lowering dropout charges. Decentralized medical trials use applied sciences resembling wearable units, affected person apps, smartphones, and telemedicine to speed up recruitment, cut back dropout, and reduce the carbon footprint of medical analysis. AWS can play a key function in enabling quick implementation of those decentralized medical trials.
On this publish, we talk about learn how to use AWS to assist a decentralized medical trial throughout the 4 important pillars of a decentralized medical trial (digital trials, customized affected person engagement, patient-centric trial design, and centralized information administration). By exploring these AWS powered options, we purpose to exhibit how organizations can drive progress in the direction of extra environmentally pleasant medical analysis practices.
The problem and influence of sustainability on medical trials
With the rise of greenhouse gasoline emissions globally, discovering methods to grow to be extra sustainable is shortly turning into a problem throughout all industries. On the similar time, world well being consciousness and investments in medical analysis have elevated on account of motivations by main occasions just like the COVID-19 pandemic. As an illustration, in 2021, we noticed a big improve in consciousness of medical analysis research searching for volunteers, which was reported at 63% in comparison with 54% in 2019 by Utilized Medical Trials. This implies that the COVID-19 pandemic introduced elevated consideration to medical trials among the many public and magnified the significance of together with various populations in medical analysis.
These medical analysis trials research new checks and coverings whereas evaluating their results on human well being outcomes. Individuals typically volunteer to participate in medical trials to check medical interventions, together with medicine, organic merchandise, surgical procedures, radiological procedures, units, behavioral therapies, and preventive care. The rise of medical trials presents a significant sustainability problem—they’re typically not sustainable and might contribute considerably to greenhouse gasoline emissions on account of how they’re being carried out. The principle sources of those are often related to the intensive power use related to analysis premises and air journey.
This publish discusses a substitute for medical trials—by decentralizing medical trials, we will cut back the main greenhouse gasoline emissions brought on by human actions current in medical trials right now.
The CRASH trial case research
We will additional look at the influence of carbon emissions related to medical trials by the carbon audit of the CRASH trial case lead by medical analysis journal, BMJ. The CRASH trial was a medical trial carried out from 1999–2004 and recruited sufferers from 49 nations within the span of 5 years. Within the research, the impact of intravenous corticosteroids (a drug produced by Pfizer) on loss of life inside 14 days in 10,008 adults with clinically vital head accidents was examined. BMJ carried out an audit on the whole emissions of greenhouse gases that had been produced by the trials and calculated that roughly 126 metric tons (carbon dioxide equal) was emitted throughout a 1-year interval. Over a 5-year interval, it might imply that all the trial could be answerable for about 630 metric tons of carbon dioxide equal.
A lot of those greenhouse gasoline emissions will be attributed to journey (resembling air journey, lodge, conferences), distribution related for medicine and paperwork, and electrical energy utilized in coordination facilities. In response to the EPA, the common passenger car emits about 4.6 metric tons of carbon dioxide per 12 months. As compared, 630 tons of carbon dioxide could be equal to the annual emissions of round 137 passenger automobiles. Equally, the common US family generates about 20 metric tons of carbon dioxide per 12 months from power use. 630 tons of carbon dioxide would even be equal to the annual emissions of round 31 common US houses. 630 tons of carbon dioxide already represents a really substantial quantity of greenhouse gasoline for one medical trial. In response to sources from authorities databases and analysis establishments, there are round 300,000–600,000 medical trials carried out globally annually, amplifying this influence by a number of hundred thousand instances.
Medical trials vs. decentralized medical trials
Decentralized medical trials current alternatives to deal with the sustainability challenges related to conventional medical trial fashions. As a byproduct of decentralized trials, there are additionally enhancements within the affected person expertise by lowering their burden, making the method extra handy and sustainable.
At this time, medical trials can contribute considerably to greenhouse gasoline emissions, primarily by power use in analysis amenities and air journey. In distinction to the energy-intensive nature of centralized trial websites, the distributed nature of decentralized medical trials affords a extra sensible and cost-effective method to implementing renewable power options.
For centralized medical trials, many are carried out in energy-intensive healthcare amenities. Conventional trial websites, resembling hospitals and devoted analysis facilities, can have excessive power calls for for tools, lighting, and local weather management. These amenities typically depend on regional or nationwide energy grids for his or her power wants. Integrating renewable power options in these amenities will also be expensive and difficult, as a result of it could possibly contain vital investments into new tools, renewable power initiatives, and extra.
In decentralized medical trials, the discount in infrastructure and onsite assets will permit for a decrease power demand general. This, in flip, will lead to advantages resembling simplified trial designs, diminished paperwork, and fewer human journey required for video conferencing. Moreover, the extra appointments required for medical trials would possibly create further time and monetary burdens for individuals. Decentralized medical trials can cut back the burden on sufferers for in-person visits and improve affected person retention and long-term follow-up.
Core pillars on how AWS can energy sustainable decentralized medical trials
AWS prospects have developed confirmed options that energy sustainable decentralized medical trials. SourceFuse is an AWS accomplice that has developed a cellular app and internet interface that allows sufferers to take part in decentralized medical trials remotely from their houses, eliminating the environmental influence of journey and paper-based information assortment. The platform’s cloud-centered structure, constructed on AWS companies, helps the scalable and sustainable operation of those distant medical trials.
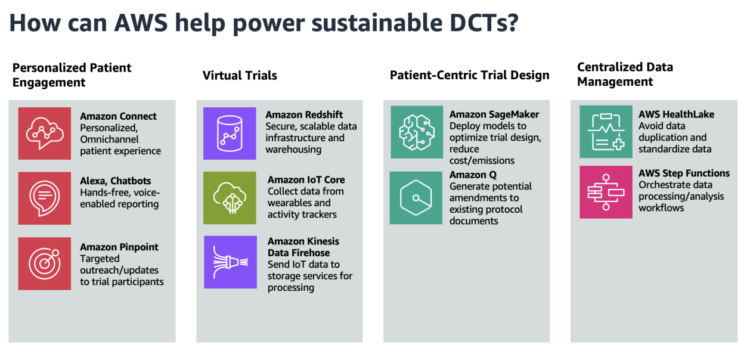
On this publish, we offer sustainability-oriented steerage centered on 4 key areas: digital trials, customized affected person engagement, patient-centric trial design, and centralized information administration. The next determine showcases the AWS companies that may assist in these 4 areas.
Personalised distant affected person engagement
The common dropout charge for medical trials is 30%, so offering an omnichannel expertise for topics to work together with trial facilitators is crucial. As a result of decentralized medical trials present flexibility for sufferers to take part at house, the expertise for sufferers to gather and report information must be seamless. One answer is to make use of voice purposes to allow affected person information reporting, utilizing Amazon Alexa and Amazon Join. For instance, a affected person can report signs to their Amazon Echo gadget, invoking an automatic affected person outreach scheduler utilizing Amazon Join.
Trial facilitators also can use Amazon Pinpoint to attach with prospects by a number of channels. They’ll use Amazon Pinpoint to ship treatment reminders, automate surveys, or push different communications with out the necessity for paper mail supply.
Digital trials
Decentralized medical trials cut back emissions in comparison with common medical trials by eliminating the necessity for journey and bodily infrastructure. As a substitute, a core part of decentralized medical trials is a safe, scalable information infrastructure with sturdy information analytics capabilities. Amazon Redshift is a totally managed cloud information warehouse that trial scientists can use to carry out analytics.
Medical Analysis Organizations (CROs) and life sciences organizations also can use AWS for cellular gadget and wearable information seize. Sufferers, within the consolation of their very own house, can acquire information passively by wearables, exercise trackers, and different sensible units. This information is streamed to AWS IoT Core, which may write information to Amazon Information Firehose in actual time. This information can then be despatched to companies like Amazon Easy Storage Service (Amazon S3) and AWS Glue for information processing and perception extraction.
Affected person-centric trial design
A key attribute of decentralized medical trials is patient-centric protocol design, which prioritizes the sufferers’ wants all through all the medical trial course of. This includes patient-reported outcomes and infrequently implement versatile participation, which may complicate protocol growth and necessitate extra in depth regulatory documentation. This will add days and even weeks to the lifespan of a trial, resulting in avoidable prices. Amazon SageMaker permits trial builders to construct and prepare machine studying (ML) fashions that cut back the chance of protocol amendments and inconsistencies. Fashions will also be constructed to find out the suitable pattern measurement and recruitment timelines.
With SageMaker, you possibly can optimize your ML surroundings for sustainability. Amazon SageMaker Debugger offers profiler capabilities to detect under-utilization of system assets, which helps right-size your surroundings and keep away from pointless carbon emissions. Organizations can additional cut back emissions by selecting deployment areas close to renewable power initiatives. At present, there are 22 AWS information middle areas the place 100% of the electrical energy consumed is matched by renewable power sources. Moreover, you should utilize Amazon Q, a generative AI-powered assistant, to floor and generate potential amendments to keep away from costly prices related to protocol revisions.
Centralized information administration
CROs and bio-pharmaceutical firms are striving to realize end-to-end information linearity for all medical trials inside a corporation. They need to see traceability throughout the board, whereas attaining information harmonization for regulatory medical trial guardrails. The pipeline method to information administration in medical trials has led to siloed, disconnected information throughout a corporation, as a result of separate storage is used for every trial. Decentralized medical trials, nonetheless, typically make use of a singular information lake for all of a corporation’s medical trials.
With a centralized information lake, organizations can keep away from the duplication of information throughout separate trial databases. This results in financial savings in storage prices and computing assets, in addition to a discount within the environmental influence of sustaining a number of information silos. To construct a knowledge administration platform, the method may start with ingesting and normalizing medical trial information utilizing AWS HealthLake. HealthLake is designed to ingest information from numerous sources, resembling digital well being information, medical imaging, and laboratory outcomes, and robotically remodel the information into the industry-standard FHIR format. This medical voice utility answer constructed totally on AWS showcases some great benefits of having a centralized location for medical information, resembling avoiding information drift and redundant storage.
With the normalized information now accessible in HealthLake, the subsequent step could be to orchestrate the assorted information processing and evaluation workflows utilizing AWS Step Capabilities. You need to use Step Capabilities to coordinate the combination of the HealthLake information right into a centralized information lake, in addition to invoke subsequent processing and evaluation duties. This might contain utilizing serverless computing with AWS Lambda to carry out event-driven information transformation, high quality checks, and enrichment actions. By combining the facility highly effective information normalization capabilities of HealthLake and the orchestration options of Step Capabilities, the platform can present a sturdy, scalable, and streamlined method to managing decentralized medical trial information inside the group.
Conclusion
On this publish, we mentioned the crucial significance of sustainability in medical trials. We supplied an outline of the important thing distinctions between conventional centralized medical trials and decentralized medical trials. Importantly, we explored how AWS applied sciences can allow the event of extra sustainable medical trials, addressing the 4 important pillars that underpin a profitable decentralized trial method.
To be taught extra about how AWS can energy sustainable medical trials to your group, attain out to your AWS Account representatives. For extra details about optimizing your workloads for sustainability, see Optimizing Deep Studying Workloads for Sustainability on AWS.
References
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1839193/
[3] https://pubmed.ncbi.nlm.nih.gov/15474134/
[4] ClinicalTrials.gov and https://www.iqvia.com/insights/the-iqvia-institute/experiences/the-global-use-of-medicines-2022
[6] https://pubmed.ncbi.nlm.nih.gov/39148198/
Concerning the Authors
 Sid Rampally is a Buyer Options Supervisor at AWS driving GenAI acceleration for Life Sciences prospects. He writes about matters related to his prospects, specializing in information engineering and machine studying. In his spare time, Sid enjoys strolling his canine in Central Park and enjoying hockey.
Sid Rampally is a Buyer Options Supervisor at AWS driving GenAI acceleration for Life Sciences prospects. He writes about matters related to his prospects, specializing in information engineering and machine studying. In his spare time, Sid enjoys strolling his canine in Central Park and enjoying hockey.
 Nina Chen is a Buyer Options Supervisor at AWS specializing in main software program firms to leverage the facility of the AWS cloud to speed up their product innovation and development. With over 4 years of expertise working within the strategic Impartial Software program Vendor (ISV) vertical, Nina enjoys guiding ISV companions by their cloud transformation journeys, serving to them optimize their cloud infrastructure, driving product innovation, and ship distinctive buyer experiences.
Nina Chen is a Buyer Options Supervisor at AWS specializing in main software program firms to leverage the facility of the AWS cloud to speed up their product innovation and development. With over 4 years of expertise working within the strategic Impartial Software program Vendor (ISV) vertical, Nina enjoys guiding ISV companions by their cloud transformation journeys, serving to them optimize their cloud infrastructure, driving product innovation, and ship distinctive buyer experiences.